Celebrating One Year of Progress: Polymotion Hip Resurfacing IDE Study
- JointMedica Admin
- Jan 22
- 2 min read
Today marks the one-year anniversary of the first Polymotion® Hip Resurfacing implant surgery conducted as part of the U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) study, a pivotal milestone in the development of this next-generation hip preservation technology.

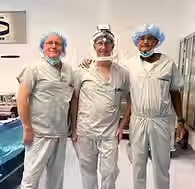
The first IDE study procedure was completed on January 22, 2025, by Mr Ronan Treacy and Dr William Peckett at King Edward VII Memorial Hospital (Bermuda). This inaugural case marked the clinical debut of the Polymotion® Hip Resurfacing System, following years of development, rigorous pre-clinical testing, and close collaboration with leading orthopaedic surgeons and regulatory experts.
The IDE study has been designed to evaluate the safety and performance of our Polymotion® Hip Resurfacing system a metal-on-polyethylene hip resurfacing device aimed at providing a bone-conserving alternative for younger, and more active patients. By moving away from contemporary metal-on-metal bearings, we are working to expand access to resurfacing for a broader patient population, particularly for women and other patients historically excluded from these procedures.
Over the past year, the IDE study has continued to build strong momentum, reflecting high levels of surgeon engagement and patient interest. The study is now 90% complete, with over 200 patients participating across ten clinical centres nationwide a powerful endorsement of the clinical community’s confidence in the technology and its potential to reshape hip resurfacing.
JointMedica remains deeply grateful to its study partners and surgical investigators for their dedication to advancing hip resurfacing science. Most importantly, we thank the patients who have entrusted us with our technology. As the IDE trial approaches completion and longer-term outcomes emerge, JointMedica looks forward to sharing further updates as Polymotion® advances toward its next major milestones.
For More Information
To learn more about the Polymotion® Hip Resurfacing System and the IDE study, please contact: cs@jointmedica.com
%20(1)_edited.png)


Comments